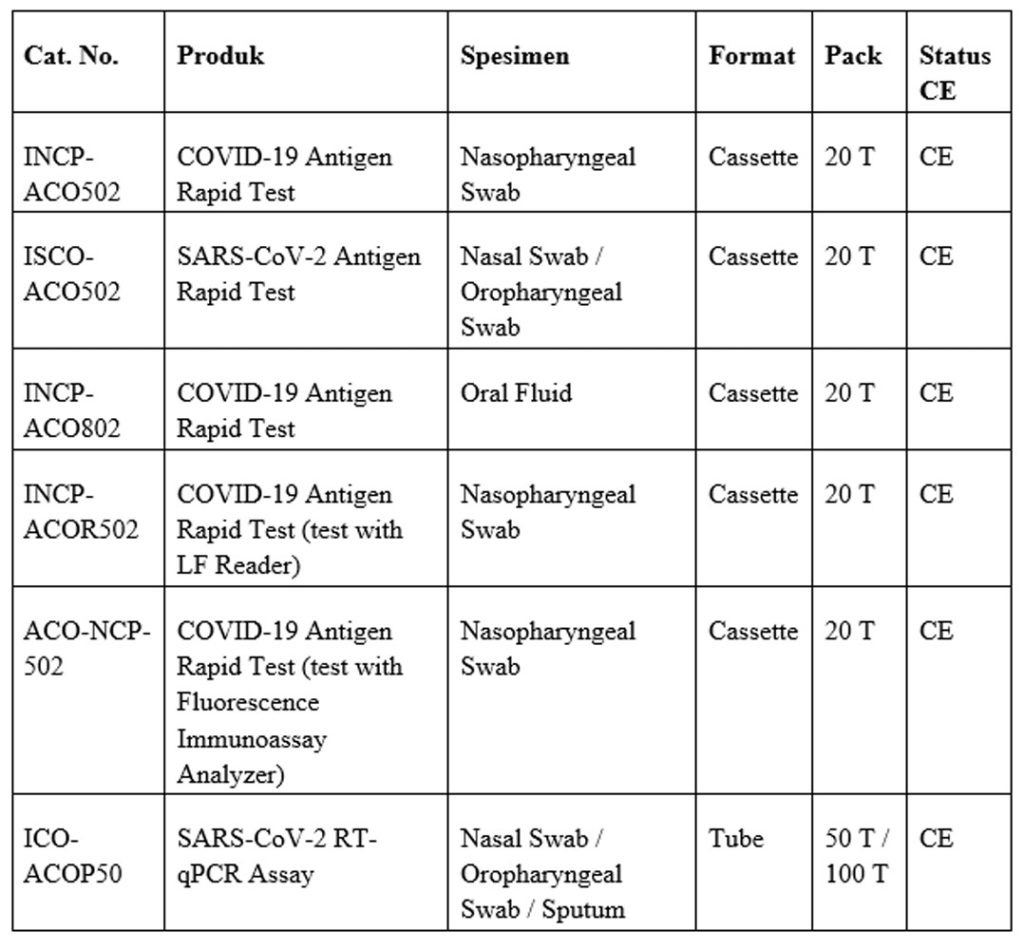
COVID-19 Rapid Tests
COVID-19 Rapid Tests are in vitro diagnostic (IVD) devices designed for the fast detection of SARS-CoV-2 infection. These tests typically use lateral flow immunoassay technology to identify specific viral antigens or antibodies in human samples such as nasal swabs, nasopharyngeal swabs, or blood/serum.
COVID-19 Rapid Tests provide reliable results within minutes, making them suitable for point-of-care testing in hospitals, clinics, laboratories, and community screening settings. Their ease of use, minimal equipment requirements, and rapid turnaround time support timely clinical decision-making, infection control, and large-scale screening programs.
These tests are widely used to aid in early diagnosis, monitor disease spread, and support public health efforts during outbreak management.

Reviews
There are no reviews yet.